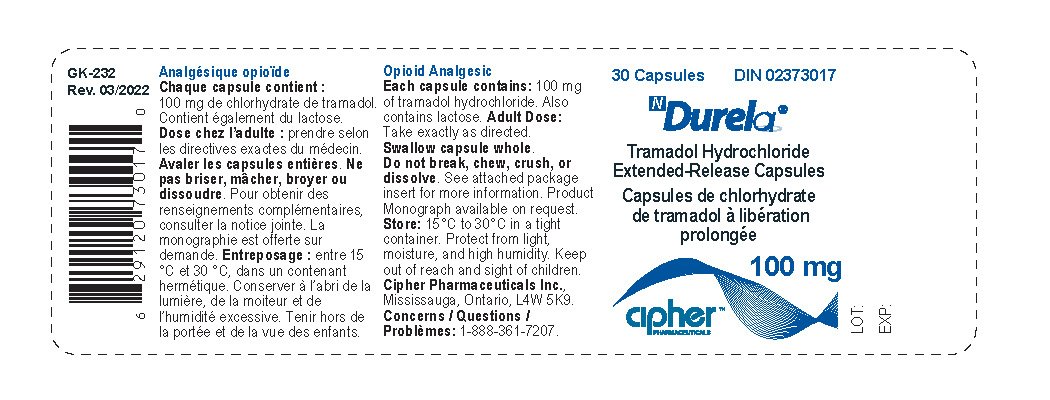
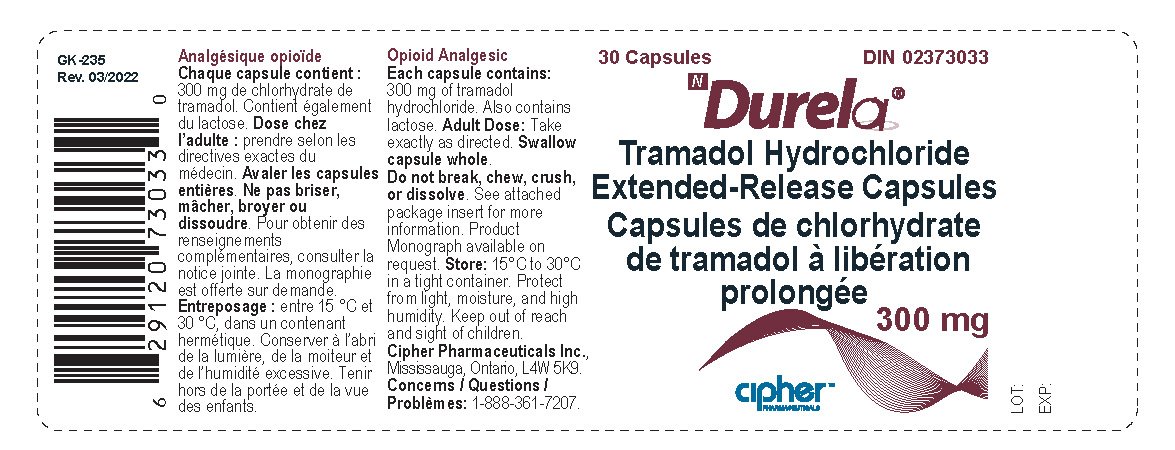
Durela Prescribing Information
Package insert / product label
Generic name: tramadol hydrochloride
Dosage form: capsule, extended release
Drug class: Opioids (narcotic analgesics)
Medically reviewed by Drugs.com. Last updated on Dec 21, 2022.
| DURELA
tramadol hydrochloride capsule, extended release |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| DURELA
tramadol hydrochloride capsule, extended release |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| DURELA
tramadol hydrochloride capsule, extended release |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - Galephar Pharmaceutical Research Inc. (003551624) |
| Registrant - Galephar Pharmaceutical Research Inc. (003551624) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Galephar Pharmaceutical Research Inc | 003551624 | analysis(66277-239, 66277-241, 66277-242) , label(66277-239, 66277-241, 66277-242) , manufacture(66277-239, 66277-241, 66277-242) , pack(66277-239, 66277-241, 66277-242) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Galephar Pharmaceutical Research Inc. | 968996160 | analysis(66277-239, 66277-241, 66277-242) | |
Related/similar drugs
gabapentin, acetaminophen, cyclobenzaprine, tramadol, naproxen, oxycodone, Tylenol
Frequently asked questions
- Can you take tramadol with acetaminophen, ibuprofen, or aspirin?
- How much tramadol should I give my dog?
- Is tramadol stronger than codeine?
- How long does withdrawal last?
- How long does it take to start working?
- How long does it stay in your system?
- Can you take 800mg ibuprofen with 50mg tramadol?
- Which drugs cause opioid-induced constipation?
- Is it an anti-inflammatory drug?
More about tramadol
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (1,839)
- Drug images
- Latest FDA alerts (4)
- Side effects
- Dosage information
- Patient tips
- During pregnancy
- Support group
- Drug class: Opioids (narcotic analgesics)
- Breastfeeding
Patient resources
Professional resources
- TraMADol monograph
- Tramadol Capsules (FDA)
- Tramadol ER (FDA)
- Tramadol Oral Solution (FDA)
- Tramadol Tablets (FDA)
Other brands
Ultram, Ultram ER, ConZip, Qdolo